Precursor Chemistry
Chemical processing of materials has played a crucial role in the development of nanomaterials science and engineering particularly due to the intimate relationship between the chemical ingredients (so-called precursors) and composition, microstructure and properties of the resulting materials. Moreover, the potential of precursor-based materials synthesis lies not only in their relatively low decomposition temperature but also more importantly in the application of chemical principles that allow controlling the decomposition mechanism through judicious choice of ligands and co-ligands. The research group of Prof. Mathur follows the bottom-up concept for synthesis of nanomaterials. The idea behind chemical synthesis of nanomaterials is the transformation of molecular precursors into materials under retention of structural units, which are inherent to the precursor molecule and also form an integral part of the final solid-state structure of the nanomaterial. The work focusses on the one hand on the synthesis of homo- and heteroleptic mono- and multimetalic alkoxide precursor for gas-phase and liquid-phase processes. The group also investigates new ligand systems with tunable properties to improve their decomposition behavior and adapt molecular precursor to the material synthesis process.
One main area of interest in our group is the preparation of functional oxide nanomaterials. To gain optimized material properties the bottom-up approach, using custom-designed precursors, outmatches in many fields the classical top-down approach using cutting, milling or shaping of the bulk material. The use of metal organic compounds as precursors facilitates in general low temperature processing of nanostructured oxide materials that are often more challenging to obtain using traditional solid-state reactions. Based on the intention to obtain very homogeneous, highly pure and crystalline multimetallic materials via solution or gas phase methods as for instance sol-gel or chemical vapor deposition (CVD) processes, the use of single-source precursors is clearly favored over a multi-source approach.
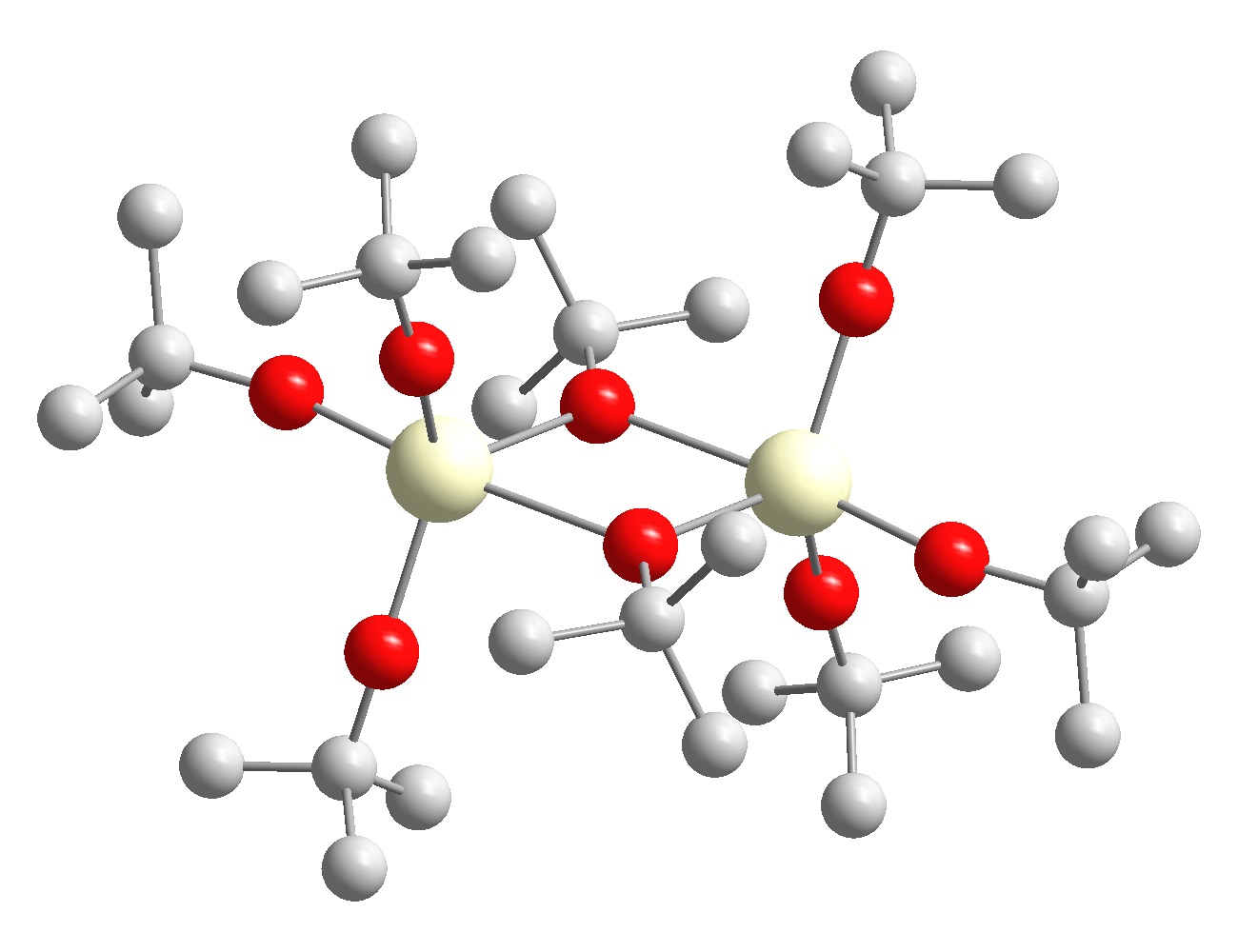
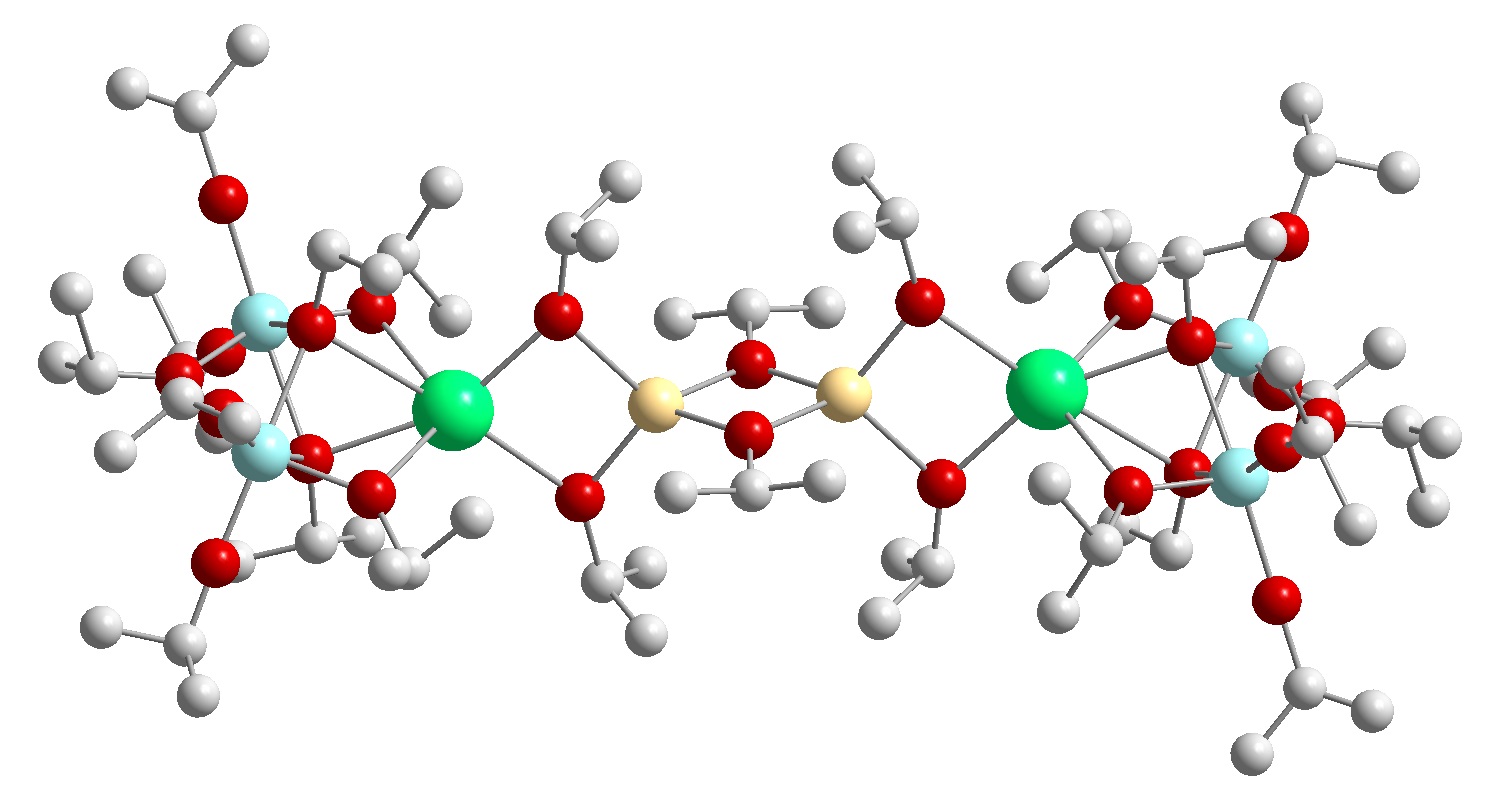
In this context, alkoxide precursors play a major role due to their structural diversity resulting from their nature to exist in different coordination or bridging modes that allow the construction of homonuclear multimetallic oligomers and also heteronuclear compounds. The general increasing interest in heterometallic frameworks can be satisfied by a smart utilization of characteristic alkoxide features as predefined M-O bonds and the coordination tendency of alkoxo oxygen atoms. These features not only provide the possibility to improve physical properties such as solubility and volatility of the new compounds by defined changes in the molecular structure of the precursor, but also enable versatile prospects for the transfer of structural information from the precursor to the final solid-state material. The predefined metal-oxygen bonds in alkoxide compounds allow the construction of a molecular template that resembles the connectivity and arrangement of elements for the targeted oxide material already on a molecular level. Induced self-assembly of these single molecule templates by controlled decomposition (CVD) or controlled hydrolysis (sol-gel) ideally results in the construction of target solid-phase materials.
Are you interested in the people behind the research?